Alumni Invents Disposable Intubation Drape
Tony Sandstrom, MSN’05, collaborated with PhD student Hideyo Tsumura, Health Innovation Lab and Center for Nursing Research for development and trial.
As a result of the COVID-19 pandemic, several products have entered the market aimed at protecting providers from respiratory droplets during airway manipulation. Tony Sandstrom, MSN’05, CRNA, noticed that, while these products generally work, they aren’t very adaptable to the provider. This observation led Sandstrom, who works at Duke University Hospital, to create the I-Drape, a disposable intubation drape.
“I always thought about things, coming up with improvements related to my profession as an anesthesia provider,” he said. “There are a few projects out there that people have thought of that look at how to protect the anesthesia provider from aerosolized particles or splatter up in the face when they are manipulating the airway, and none of them are user friendly, so to speak. I thought how about you have a completely different view of how to protect the provider.”
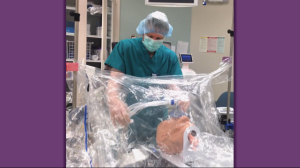
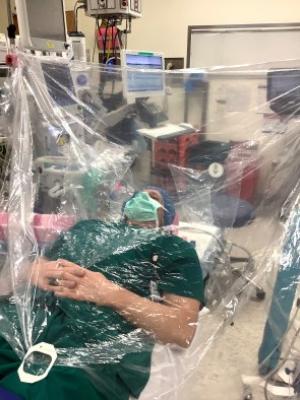
The I-Drape is made up of clear plastic, specifically medical grade, 100% polyethylene. The user would install it by securing its Velcro strips or with clips, if any are on hand, to the IV or bed poles. The user would come up to the end that is attached to the poles and slip their hands into the built-in sleeves. The other end of the drape falls over the patient’s upper body; its attachment to the poles angles the drape up away from the patient’s face. A video demonstration can be found at the end of this article.
“The anesthetist is able to access the supine patient from above the patient’s head and can proceed with the intubation using the integrated arm sleeves with gloves,” said Hideyo Tsumura, PhD student who has been involved in the product’s development and testing. “There is an integrated pocket inside the drape for storage of contaminated equipment (e.g., laryngoscope, oral airway, suction catheter, oxygen mask), and there are small tabs on the dorsal side of the glove designed to make removal of the hand from the glove easier.”
Starting with a trash bag, Sandstrom went through five or six prototypes before landing on one that fit his vision. He worked with a company in Atlanta to manufacture his prototype into a tangible product.
Currently, because of its institutional review board approval, Duke providers can implement the I-Drape into their procedures. To make it available to non-Duke providers, the product would have to approved by the FDA. Widening access to this product could benefit health care sites with limited resources.
“He thought, this is something that is cheap to make that also provides protection and can be very useful in underserved areas or underdeveloped countries,” Tsumura said.
Sandstrom was advised that running a trial on the product would help with the FDA application. That’s when Tsumura entered the process.
Tsumura consulted with the Health Innovation Lab (HIL) and the Center for Nursing Research (CNR) to help guide them through the processes to get the approval they needed to run the trial at Duke. Since Tsumura is a student, she needed to work directly with a principal investigator and chose Ryan J. Shaw, PhD, RN, associate professor and HIL director.
“He was very supportive, and he provided everything that we needed to do this,” she said, adding that Jacqueline Allen, data analysis, and Susan E. Hunter, research practice manager, from the CNR were also supportive throughout the process.
The trial took around two months to complete. The opportunity was open to nurse anesthetists, residents and fellows and resulted in approximately 30 nurse anesthetists enrolling.
Tsumura collaborated with the provider and anesthesiologist to identify potential patients for the study. The patient had to be older than 18 and could not have a difficult airway, be allergic to plastic, be pregnant or have a severe respiratory disease. Tsumura stayed available to the provider throughout the process, assisting with installation and answering any questions that arose.
“The trial actually went really, really well because we did about 59 trials, and out of 59 times, you only had two cases where we had to take down the drape, which to me is a really good success rate,” she said.
She added that the two times the drape was removed was due to the provider’s preference and not because the patient was negatively reacting to its usage. The reception was, overall, positive with the main suggestion being to adjust the drape’s fit to better suit more body types.
“It was a universal design that, across years of experience, regardless of how many times they used the device, they can successfully integrate and use this device,” Tsumura said.